Short-Term Efficacy of Using a Novel Low-Volume Bone Marrow Aspiration Technique to Treat Knee Osteoarthritis: A Retrospective Cohort Study
Kuebler D, Schnee A, Moore L, Kouri J, McLaughlin A, Hanson R, Kuebler P, Dallo I, Gobbi A.
Stem Cells Int., vol. 2022, Article ID 5394441, 7 pages, 2022 | doi: 10.1155/2022/5394441
Methods:
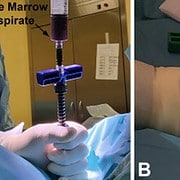
This retrospective cohort study examined the effect of using low-volume BMAs harvested using the Marrow Cellution™ (MC) device on 160 patients (262 knees) suffering from pain due to knee OA, KL grades 2-4, that did not respond to conservative treatment. Changes in visual analog scores (VAS) for overall daily activity were examined over a six-month time frame in these patients (63:5 ± 0:97 years of age; 48.1% male). In addition, changes in the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and Patient Global Impression of Change (PGIC scores) were examined over the same time frame in a smaller subset of patients (95 patients including 172 knees).
Results:
There was a statistically significant improvement in VAS scores for overall daily activity 6 months postprocedure in the study population, 7:29 ± 0:27 vs. 3:76 ± 0:34 (p < 0:0001), as well as statistically significant improvements in WOMAC scores, 49:3 ± 4:27 vs. 66:3 ± 4:08 (p < 0:0001). On the individual level, 71% of the cases displayed VAS improvements and 61% of the cases displayed WOMAC improvements that exceeded levels previous studies determined to be the minimal clinically important difference (MCID) for knee OA treatments. The improvements in WOMAC scores were also seen in both the WOMAC pain subscore, 52:2 ± 4:39 vs. 72:2 ± 4:36 (p < 0:0001) and the WOMAC function subscore, 51:6 ± 4:67 vs. 69:0 ± 4:36 (p < 0:0001). In addition, the PGIC scores measuring patient satisfaction improved from 4:03 ± 0:26 at 6 weeks postprocedure to 4:65 ± 0:28 at 6 months postprocedure (p < 0:0001).
Conclusions:
Knee OA patients treated with MC BMA intra-articular injections exhibited significant reductions in VAS pain scores and significant improvements in WOMAC scores that exceeded the minimal clinically important difference thresholds. In addition, reductions in VAS pain scores and improvements in WOMAC scores correlated with higher PGIC scores.
______________________________________________________
The Marrow Cellution™ System is designed to minimize and eliminate the restrictive limitations associated with “point of care” processing systems which employ inefficient traditional aspiration trocar technology and are regulated as drugs “Advanced Therapies Medicinal Products (ATMP)”. Marrow Cellution™ is a minimally invasive bone/cell enriched autograft access and retrieval system, which does not require additional manipulation or processing, it never leaves the sterile field and provides rich autograft without associated morbidity.
Visit our Marrow Cellution product page





 Matrix Biosurgical Inc.
Matrix Biosurgical Inc.
