Technique Matters: Small Draw, Diverse Geography, Precise Repositioning, Lateral Draws Only, Pace, and Syringe Plunger Yields Clinically Relevant CFU-f Counts
Paul D Tortland, Daniel Kuebler.
Pilot Study | White Paper
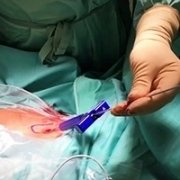
ABSTRACT: Positive clinical outcomes for orthopedic treatments utilizing autologous bone marrow-derived stem cells have been linked to the cellular content of the marrow aspirate graft as measured by fibroblast-like colony-forming units (CFU-f ) as certain minimum thresholds have been associated with beneficial outcomes. The purpose of this study was to examine if a modified technique could produce a graft in which the CFU-f content exceeded those thresholds. The modified technique utilized the Marrow Cellution™ device (MC), which aspirates small aliquots of marrow across a large geography from the posterior iliac spine, and aspirating solely from the side ports of the harvesting device.
In this pilot study, we investigated the cellular recovery using the MC device by altering the published protocol by reducing the volume of aspirate to only 5mL across the first half of the geographic trajectory before switching the syringe to aspirate another 3mL to 5mL per location across the remaining geographic trajectory. Stem/progenitor cell concentrations as counted by CFU-f and TNC (total nucleated cells) were performed on a 1mL sample taken from the first syringe containing 5mL of native aspirate. These tests represent a standard to determine the number of immature stem and progenitor cells that are present in the aspirate.